Recall of Specific Batches of EleCare Similac and Alimentum Similac due to the Possible Presence of Salmonella and Cronobacter sakazakii
Monday, 21 February 2022
| Alert Summary | |
|---|---|
| Category 1: | For Action |
| Alert Notification: | 2022.14 |
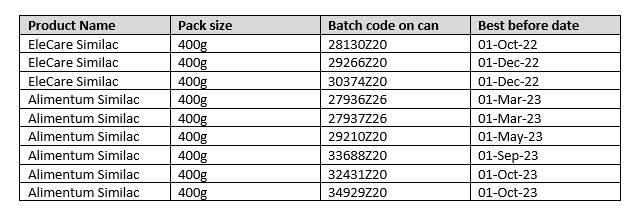
| Product Identification: | Please see table below |
| Batch Code | Please see table below |
| Country Of Origin: | USA |
Message:
Abbott is recalling specific batches of its EleCare Similac and Alimentum Similac due to the possible presence of Salmonella and Cronobacter sakazakii. No products distributed to Ireland have tested positive for the presence of Cronobacter sakazakii or Salmonella Newport. Both products are foods used for special medical purposes for infants, ordinarily to be used under medical supervision. Point-of-sale recall notices will be displayed in stores supplied with the implicated batches.
Nature Of Danger:
Salmonella and Cronobacter sakazakii can cause illness in infants if they are present in powdered infant formula. Although Cronobacter sakazakii and Salmonella cannot grow in powdered infant formula, they can survive for a long period of time and therefore, pose a potential risk after rehydration if the product is temperature abused. Contamination of powdered infant formula with Cronobacter sakazakii and Salmonella can cause severe disease in infants such as diarrhoea (sometimes bloody), fever, sepsis or meningitis which can lead to serious neurological and developmental issues and can be fatal on rare occasions. Sepsis and meningitis may include poor feeding, irritability, temperature changes, jaundice (yellow skin and whites of the eyes) and abnormal breaths and movements. Among infants, those at greatest risk for infection are neonates (<28 days), particularly pre-term infants, low-birth weight infants or immunocompromised infants.
Action Required:
Manufacturers, wholesalers, distributors, caterers & retailers:
Retailers are requested to remove the implicated batches from sale and to display a point-of-sale recall notice in stores where the affected batches were sold.
Consumers:
Parents and guardians are advised not to feed the implicated batches to infants.