Recall of GreenHeart CBD Oils due to the Presence of Unsafe Levels of Delta‐9‐tetrahydrocannabinol (THC)
Wednesday, 23 February 2022
| Alert Summary | |
|---|---|
| Category 1: | For Action |
| Alert Notification: | 2022.15 |
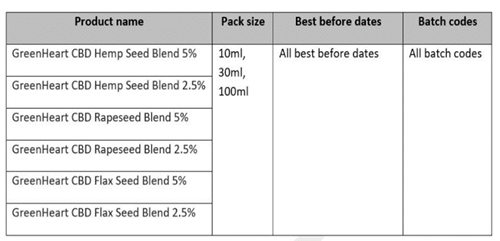
| Product Identification: | See table below for product detail |
| Batch Code | All batches and all best before date |
| Country Of Origin: | Ireland |
Message:
All batches of GreenHeart CBD oils are being recalled due to the presence of unsafe levels of delta‐9‐tetrahydrocannabinol (THC). Also, the below batches contain lower levels of CBD than that declared on the product, which is misleading to the consumer.
Nature Of Danger:
The implicated batches of Greenheart CBD oils listed below contain unsafe levels of delta‐9‐tetrahydrocannabinol (THC) based on the European Food Safety Authority (EFSA) acute reference dose.
Action Required:
Consumers:
Consumers are advised not to consume the implicated products.